
Research Gate: Pharmaceutical Science
The Research Gate: Pharmaceutical Science provides a resource content dealing with the Pharmaceutical industry starting from drug discovery process to drug distribution system to patients.
The Research Gate: Pharmaceutical Science aims to publish all the recent and exceptional research articles and reviews in all areas of modern Pharmaceutical industry like drug discovery including in-silico drug design, combinatorial chemistry, new drug targets, Bioinformatics and chemoinformatics, Genomics and proteomics, medicinal chemistry, SAR, high-throughput screening, advances in ADME, drug delivery and Biopharmaceuticals, phytochemistry and pharmacognosy.
Journal Policy
Current Issue: Research Gate: Pharmaceutical Sciences - July - Sep 2025
Volume 14, Number 3 (2025)
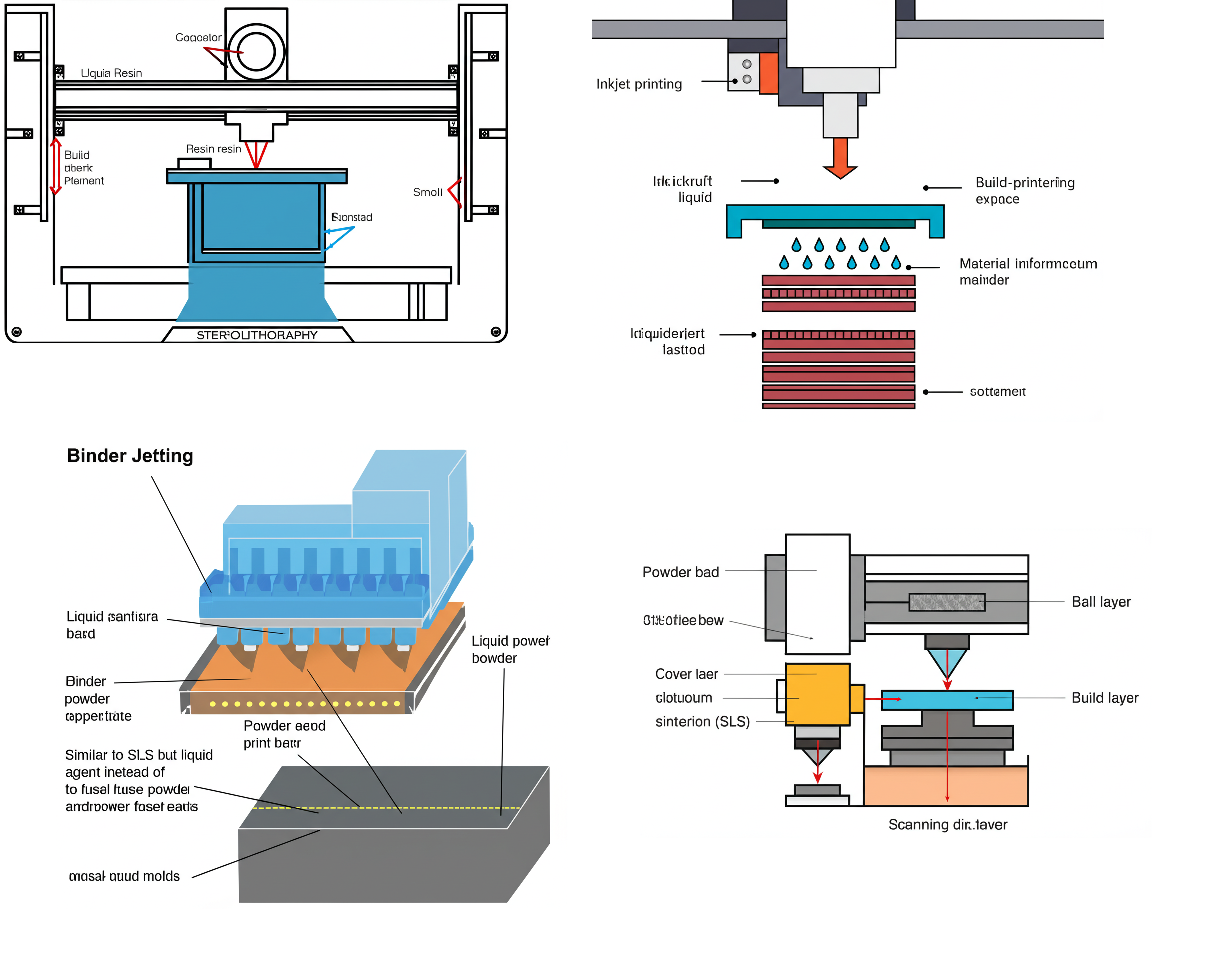
Personalized Medicine: 3D Printing for Tailored Drug Dosage - A Review
By M. PRIYA LAKSHMI
3D printing holds immense potential to revolutionize personalized medicine by enabling tailored drug dosages, improving patient outcomes, and reducing side effects. This technology is transforming drug manufacturing, supply chains, and regulatory landscapes, opening up new opportunities for innovation and investment. 3D printing is poised to transform healthcare by enabling personalized drug dosages, leading to improved patient outcomes, reduced side effects, and a more efficient healthcare system. The future of 3D printing in personalized medicine is bright, with emerging technologies like 4D printing, bioprinting, and AI promising to further enhance the personalization and efficacy of drug delivery. Researchers, clinicians, regulators, and industry stakeholders must collaborate to accelerate the development and implementation of 3D printing for personalized medicine. This collaboration is essential to unlock the full potential of this transformative technology. 3D printing has the power to create a more personalized, efficient, and patient-centric healthcare system, where medications are tailored to individual needs, improving health outcomes and quality of life for patients worldwide.

Navigating Regulatory Challenges: A Comparative Exploration of Regulatory Missteps in Pharma"
By S .Naveen Kumar
This article explores global regulatory challenges in the pharmaceutical industry using a comparative case study approach. It examines incidents involving pricing violations, product recalls, and regulatory enforcement across different regions, identifying key patterns of non-compliance and operational gaps. The analysis highlights common issues such as inadequate quality systems, poor documentation practices, and lack of regulatory preparedness. Emphasis is placed on building a culture of compliance, ensuring patient safety, and implementing proactive risk management strategies .By drawing practical insights from real-world events, the article offers guidance for regulatory professionals, quality assurance teams, and decision-makers aiming to strengthen compliance frameworks and navigate the evolving global landscape of pharmaceutical regulation.
News & Announcements
There are no news or announcements at this time.
